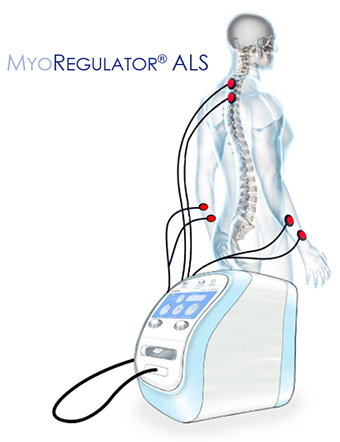
MyoRegulator® ALS
MyoRegulator® ALS is our company’s next-generation product, and is expected to be the first neuromodulation device for the treatment of ALS disease progression. ALS is a progressive neurodegenerative disease that affects motor neurons in spinal cord and brain, resulting in muscle weakness, paralysis and eventual death. Motor neuron hyperexcitability and protein aggregation are hallmarks of the ALS disease process, and our published work in NKCC1 (Na-K-Cl cotransporter) suppression initially led us to the application of our technology to ALS. At present, there are only 2 FDA-approved small molecule drugs and 1 FDA-approved anti-sense oligo (ASO) for the treatment of ALS, all having limited effectiveness. As such, there is tremendous unmet medical need in the ALS field, and we are working to urgently bring an entirely new treatment modality to this unrelenting and terrible disease.
MyoRegulator® ALS has emerged from our proprietary technology platform for Multi-Site DCS, and provides non-invasive multi-level spinal stimulation together with bilateral peripheral stimulation. Importantly, this product is being developed for at-home use, and will enable therapy to be delivered in the comfort and privacy of the home. We have developed a robust package of pre-clinical data that has been presented at leading ALS meetings, and includes electrophysiology, biochemistry, motor function and survival studies in the gold standard ALS models. We have been able to advance our work in ALS with the tremendous support of the National Institutes of Health (NIH), Cullen Education and Research Fund (CERF), Muscular Dystrophy Association (MDA) and U.S. Department of Defense (DoD), and are actively working with leading clinical collaborators to conduct a second, larger human clinical trial of our prototype device in individuals with ALS beginning in early 2025.