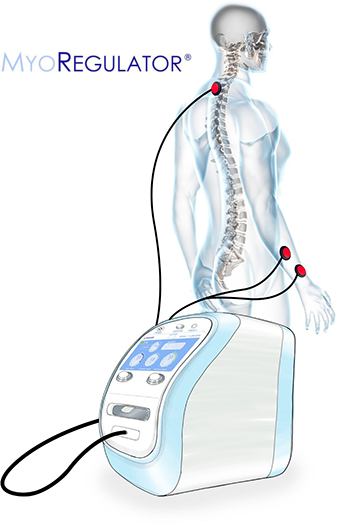
MyoRegulator® PM-2200
The MyoRegulator® PM-2200 system provides a novel treatment modality for muscle spasticity based on non-invasive neuromodulation. Spasticity is a common condition seen in many people suffering from stroke, ALS, cerebral palsy, multiple sclerosis, spinal cord injury, traumatic brain injury and other conditions. Management of spasticity is currently a difficult and sometimes insurmountable challenge, and there is tremendous unmet medical need. Pharmacological, surgical, and physical treatments to manage spasticity have, at best, short-term efficacy, are confounded by adverse effects, and are often unpleasant for the individual being treated. In US and other countries, repeated intramuscular injections of botulinum toxin have been approved for use as a treatment for spasticity, though toxicity issues and serious adverse effects have been reported (1,2). Furthermore, the cost of expensive botulinum toxin is becoming more prohibitive in this current era of cost-containment.
MyoRegulator® is based on our proprietary DoubleStim® technology, which provides simultaneous non-invasive stimulation at spinal and peripheral sites, resulting in suppression of hyperexcitable spinal circuits found in people with spasticity. MyoRegulator® has FDA “Breakthrough Device” designation and FDA has confirmed that clinical trials with MyoRegulator® are considered Non-Significant Risk (NSR). Early feasibility clinical use of our DoubleStim® technology has demonstrated promising results in treating spasticity related to stroke and cerebral palsy. An IRB-approved European pivotal trial in post-stroke spasticity has been successfully completed, and a US multi-center pivotal trial funded by NIH has also concluded. The MyoRegulator® device is also being evaluated in two clinical trials for people with ALS.
¹ Phadke et al., “Adverse Clinical Effects of Botulinum Toxin Intramuscular Injections for Spasticity”, Can J Neurol Sci. 2016; 43:298-310
² Yiannakopoulou, “Serious and Long-Term Adverse Events Associated with the Therapeutic and Cosmetic Use of Botulinum Toxin”, Pharmacology 2015; 95:65-69